One of the emerging markets in the pharmaceutical industry, the pediatric medicines market requires a huge amount of investments and R&D activities. One major reason behind the growing demand for pediatric medicines is the fact that the birth rate has increased rapidly – both in the developed as well as the developing nations. Also with changes in the environment, and incidences of delayed pregnancies, infants are more prone to develop health-related complications. Infants and kids have emerged as a major consumer group for several pharma companies.
Potential equals profits in America
When compared to APAC and EMEA, it is the Americas which have a huge potential for pediatric medicines. The fact that infectious diseases and respiratory disorders are quite common among kids and infants in this region is another major driving factor for the growing demand for pediatric medicine. Holding a little under 50% of the global pediatric medicines market, this region has the advantage of having the proper R&D institutes and policy framework in place.
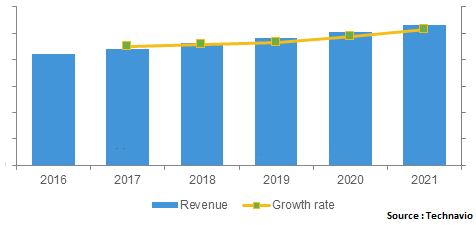 Pediatric medicines market in Americas 2016-2021 ($ billions)
Pediatric medicines market in Americas 2016-2021 ($ billions)
In a major push to the pediatrics medicines market, the National Institute of Health (NIH) has initiated Best Pharmaceuticals for Children Acts (BPCA) with the aim to improve the R&D of Paediatric therapeutics.
The major aims of this act include:
- To identify the drugs, which require more clinical study as there is a lack of data related to the dose, safety, and efficacy of the same
- To provide financial help for these trials
- To monitor the effects of these drugs on children
- Data submission to the Food and Drug Administration (FDA) for label modification
- To identify various sites to conduct trials through a competitive peer review process
Also, high incidence of industry-academia collaboration has broadened the product line of paediatric drugs. Therefore, it is safe to conclude that Americas is the next big hub for the global pediatric medicines market.
Potential roadblocks : What must vendors be wary of?
One major challenge which players in the pediatric medicines market face is the lack of scope for clinical trials. As the trial patient pool is quite limited, the chances of approval for new drugs is quite low. Apart from ethical issues, physiological, and pharmacometrics issues are the other factors which have emerged as a problem in the global pediatric medicines market.
Also, use of off-label drugs is another major challenge. Our market research report shows that around 80% of hospitalized pediatric patients are prescribed off-label drugs. These drugs do have a negative impact on the health of the infants and kids. Thus, the need of the hour is to formulate a better policy framework which encourages R&D activities and curtails the rampant prescription of off-label drugs.



