From what was first introduced as a bailout technique for failed or suboptimal iliac angioplasty to a versatile and valuable instrument for an assortment of primary and adjuvant applications, the technology in vascular stents industry has progressively evolved, thanks to the emergence of coronary and peripheral vascular stents, delivering precision with a range of innovative products.
However, the question remains constant for patients as to which stent is better and how long can a stent stay in their bodies. Associatively, there have been several issues facing companies to come up with products that can deliver high accuracy and precision along with affordability. Thus far, many products have been introduced that promise to perform better than their predecessors.
Search for the ideal vascular stent : Long lasting, affordable and maintenance free
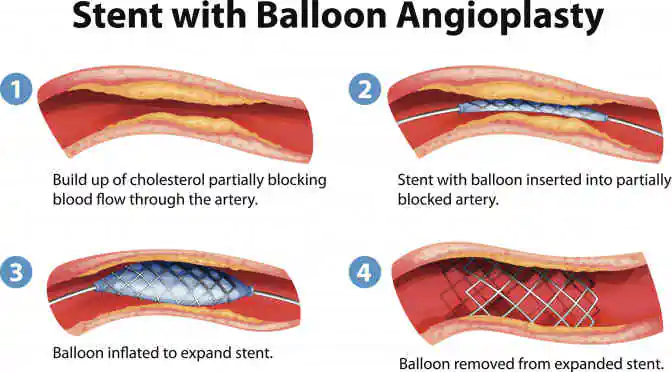
Until the arrival of coronary stents, patients with cardiovascular blockages had little choice but to undergo coronary artery bypass grafting (CABG). However, With the introduction of angioplasty and stent in 1977, patients were given a less complicated option to open blocked coronary arteries. Nevertheless, this method required repeated intervention.
 In an effort to lessen the frequency of patients requiring reintervention, clinicians and scientists developed a range of coronary stent products that could be left behind to hold the artery open, namely, bare metal stents (BMS), drug eluting stents (DES), and bio resorbable stent (BRS). A comparative analysis is listed below-
In an effort to lessen the frequency of patients requiring reintervention, clinicians and scientists developed a range of coronary stent products that could be left behind to hold the artery open, namely, bare metal stents (BMS), drug eluting stents (DES), and bio resorbable stent (BRS). A comparative analysis is listed below-
Bare metal stents (BMS)
BMS was the first-generation stents made of bare metals. They are typically made of stainless steel and have no special coating while acting as a scaffolding to prop open blood vessels after they are widened with angioplasty. Although this technique nearly eliminated the risk of the artery collapsing, they only modestly reduced the risk of re-blockage. About a quarter of coronary arteries treated with bare-metal stents would close again, usually in about six months.
Drug-eluting stents
Subsequently, clinicians and brands started testing stents coated with drugs that interrupted the re-blockage. These advances are called drug-eluting stents.
Drug-eluting stents were introduced to release a drug primarily intended to trim-down the incidence of restenosis. From there, several innovative products have been developed by top healthcare brands, such as bioactive stents designed to attract a patient’s endothelial cells to coat the stent.
As per the trial conducted by NCBI, compared to balloon angioplasty, BMS, and DES, DES had demonstrated incremental advances over a period delivering benefits such as lowest rates of restenosis, stent thrombosis, and recurrent myocardial infarction.
Promising leads signal the next evolution of vascular stents
In clinical trials, several innovative next-generation stent designs have been approved or being developed by top technology companies. These include:
- One with a layer that carries an anti-restenosis drug for few months and then becomes a bare-metal stent
- Another version that is absorbed by the body and dissolves after it has completed its work
In 2016, the FDA approved a novel category of stent made of a preferred polymer that ultimately dissolves into the body. This innovative technology is called an absorbable stent which releases a drug called Everolimus, which restricts the growth of scar tissue that can re-narrow the coronary artery.
The latest advances are expected to reduce the cost further and improve clinical outcomes. It will lessen the price due to a decline in the need for future procedures and hospitalizations due to recurrent coronary disease.
Opportunity amidst regulatory challenges
Despite a plethora of opportunities, the global market for vascular stents poses a bunch of serious challenges for innovators to think up new products mainly in the form of strict regulations and product recalls.
The Food and Drugs Administration (FDA) has set strict guidelines for the development of new products. The novel products must go through several trial, testing, and validation. The process to get an approval is time-consuming with big investments lying in limbo until said approvals are in force.
Consequently, failure of the trial at any stage results in huge loss of time and money for the developers. Plus, regulatory bodies have imposed strict labeling requirement, pre-market approval, and medical device reporting before launching of product. These devices are considered to be highly-risky and need to go through all the safety examinations to prove their medical efficacy before approval.
Each device category must follow its specific compliance norms; some devices need to undergo strict clinical testing before approval, whereas some may have to go through the process of extensive regulatory agency review to assess the product’s safety and efficacy.
The looming threat of product recalls
There is no doubt that medical devices must be manufactured with high precision and a slight variation in the process can lead to a defect in the equipment which can even result in the death of a patient. In the same light, vascular stents are complex and critical tools, and use of a non-compliant device can compromise with patients’ safety. There have been several instances of vascular stent products being recalled due to defects, and one of the recent examples is mentioned below:
In March 2017, Abbott initiated a product recall of Abbott NC Tenku RX PTCA Balloon Catheter, Abbott NC Trek RX Coronary Dilatation Catheter, and Abbott NC Traveler Coronary Dilatation Catheter. These products were recalled mainly due to 19 reports of injury and one report of death associated with difficulty in removing the protective balloon sheath.
Learn more about the evolving landscape of innovative vascular stents technology and gain in-depth insights into the most recent findings from Technavio’s industry experts by downloading the sample report as listed herein.