Artificial pancreas technology is an emerging and significant breakthrough in treatment options for Diabetes. And it could be available to patients in as little as two years.
An artificial pancreas is a closed-loop insulin delivery system that mimics the functions of a healthy pancreas in the human body by continuously monitoring glucose levels and automatically adjusting insulin delivery with minimal or no patient interaction. Continuous glucose monitoring systems incorporating the artificial pancreas technology have been developed.
Artificial Pancreas Gets FDA Seal of Approval 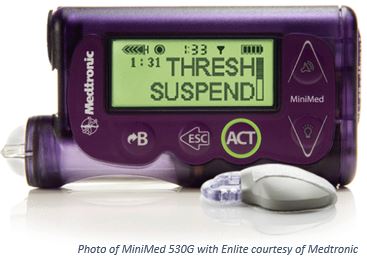
Recently, the FDA approved the MiniMed 530G with Enlite system offered by Medtronic. This is a first-generation artificial pancreas system with threshold suspend automation, a feature that automatically stops insulin delivery when glucose levels fall below a predetermined threshold.
This is especially important for the United States, where the increased prevalence of diabetes among children and adults is the cause for the increased demand for continuous glucose monitoring systems to control the glucose levels better.
- It is estimated that 25.8 million Americans had diabetes in 2013.
- 215,000 of those diagnosed were below 20 years of age.
- Nearly 3 million Americans have type 1 diabetes.
The recent FDA approval for artificial pancreas technology is expected to significantly spur the growth of the market in this region, and will likely encourage other regions to invest in artificial pancreas technology.
Artificial Pancreas Project Pipeline
Though FDA approval is a recent breakthrough, the quest to create an artificial pancreas isn’t new. In 2006, the Juvenile Diabetes Research Foundation (JDRF) commissioned the Artificial Pancreas Project (APP) to pipeline the development of a commercially viable artificial pancreas.
In a recent interview with WTHI-TV, Doctor Aaron Kowalski, a lead scientist for the JDRF, said that this artificial pancreas technology could be made available to US patients in as little as a year and a half.
“I just came back from England and was talking to a mom who is pretty much welling up as she described the experience of having her 14-year-old living at home with an artificial pancreas and how her blood sugar was perfect every morning and it just tremendously made their life easier, safer,” said Dr. Kowalski.
Concurrently, a team of scientists led by Doctor Roman Hovorka at the University of Cambridge and Cambridge University Hospitals is testing an artificial pancreas. According to a Cambridge News post, the team is looking at two major aspects:
“Improving glucose control to make it more stable and more normal. It’s going to be associated with patients with long term complications such as kidney or eye problems.
“And to reduce the risk resulting in lower glucose volumes, hyperglycaemia, when it happens at night. Especially in children, the system automatically controls the glucose during the day and night.
“Our study is a stepping stone. We’re looking beyond the study how it can progress to wider use. It’s an important study but it still needs to be followed by other investigations. It could transform and revolutionise treatment.”
Hopefully with this research, the global diabetic population will begin to see rollouts of artificial pancreas technology within the next few years.